For well over 100 years, cell biologists have known that most of the cells in our bodies, as well as those out in nature, grow to a very specific size that is remarkably consistent within their cell type. This phenomenon of growing to the “right” size is critical for their ability to carry out vital functions such as mitosis or cell division. But only in very recent years have researchers begun to understand how cells are able to accomplish this.

“A big advantage we have working in the field today are the many tools that we have at our disposal—which include genetic, genomic, biochemical, and microscopy techniques—that are allowing us to identify some of the key molecular mechanisms that are involved in cell size control,” explains James Moseley, PhD, an associate professor of biochemistry and cell biology at Dartmouth’s Geisel School of Medicine.
In the following Q&A, Moseley talks about how findings from a recent study, published in the Journal of Cell Biology by he and his research team in the Moseley Lab, are shedding new light on this complex process.
Q: For starters, can you describe your research and what your lab is focused on?
Moseley: As a cell biologist, I’m interested in how cells work, and the aspect of cell biology that we focus on is how cells coordinate both cell growth and division. At the heart of this coordination are signaling pathways that link cell growth proteins with the core cell cycle machinery. We use a multidisciplinary approach to identify these pathways, and then to understand how their activities are controlled by changes in cell size and shape.
My lab uses a model system of fungus called fission yeast—a single-celled eukaryote that grows and divides at a very reproducible size—that allows us to employ a wide range of research techniques that are hard to do in human cells.
A lot of the core elements of understanding cell cycle progression were identified decades ago in yeast, based on genetic screens. Subsequent work then determined that many of the genes and proteins that control cell division in yeast cells are the same as in human cells. So, what we’re doing now is taking those genes and the proteins that they encode and trying to understand how they all work together—to convey a more dynamic picture of when and where these interactions are happening.
Q: Can you tell us about the new study and its findings?
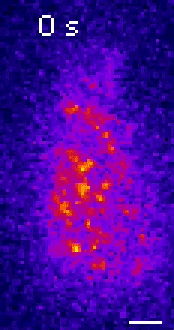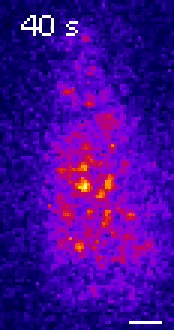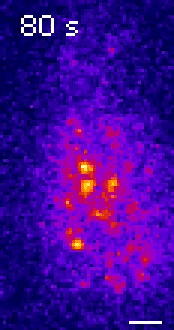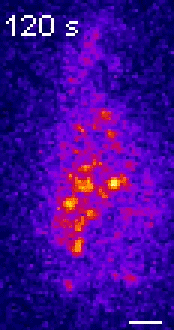
Moseley: One of the key breakthroughs we had in this paper was being able to watch a protein called Wee1. There aren’t a lot of copies of this protein floating around in a cell, which makes it really hard to see. But Corey Allard, a graduate student in the lab who led most of the work in this study, had the great insight of using a TIRF (total internal reflection fluorescence) microscope. My lab doesn’t have a TIRF microscope, but we have access to one in our core imaging facility here at Geisel. Rather than looking all the way through the cell, it allows you to look just at the plasma membrane on the very surface of the cell.
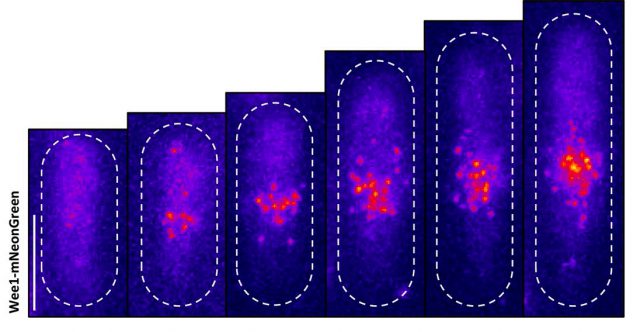
We were able to see some really interesting changes in how Wee1 goes to these spots called nodes that we’ve been studying at the cell surface as the cell gets bigger. Corey was able to capture this activity in a series of images and movies that are featured in the study.
It turns out that Wee1 transiently visits these nodes, but it does so in a very size-dependent manner—as the cells get bigger, it visits them much more frequently. And that fits perfectly with a lot of the biochemistry and genetics work that our lab has been doing to understand that Wee1 is getting inhibited or “turned off” at these structures. This is what allows the cell to enter into cell division.
Q: How is your work helping to inform efforts on the clinical front?
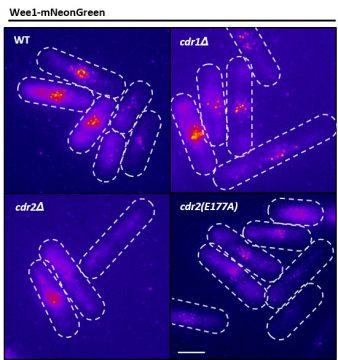
Moseley: Cancer is the most relevant disease to our work—our lab gets funding from both the government and the American Cancer Society. But what a lot of people may not realize is, much of the work that’s important to understanding cancer gets done on organisms like fission yeast.
Since basic cell growth and cell cycle systems are well conserved between fission yeast and human cells, we try to understand how the process is supposed to work correctly. We can then go in and make very specific mutations in these genes and see what goes wrong. This, I think, provides a foundation of knowledge for people who are working with the same genes and proteins in human cells and cancer cells. And certainly, some of the close collaborations that we have with other labs and the supportive environment we have here at Dartmouth greatly enhance these efforts.